Walter Russell Periodic Table: Unlocking the Secrets of the Elements

The Walter Russell Periodic Table represents an entirely new approach to understanding the periodic relationships between the elements. While the traditional periodic table, designed by Dmitri Mendeleev in 1869, has served as the foundation for chemistry for over a century, Walter Russell, a renowned American polymath, sought to rethink and refine this structure. His version is unique in that it blends the traditional concepts of chemistry with a more holistic understanding of physics, energy, and the natural world.
In this article, we’ll dive into the Walter Russell Periodic Table, exploring its origins, key differences from the traditional periodic table, and how it offers a new lens through which we can understand the elements. Whether you’re a student of science, a chemistry enthusiast, or someone curious about the deeper forces of the universe, this exploration is sure to provide fascinating insights.
Who Was Walter Russell?
Before we dive into the specifics of the Walter Russell Periodic Table, it’s important to understand who Walter Russell was and what motivated him to propose an alternative to the standard model.
Walter Russell (1871–1963) was a multi-talented figure, a self-taught scientist, philosopher, and artist. He gained recognition for his work in a variety of fields, from metallurgy and chemistry to astronomy and the arts. In the early 20th century, Russell developed several groundbreaking theories that challenged conventional scientific understanding. His most famous work, the Russell Periodic Table, was presented in 1926 as part of his broader theory of a dynamic, energy-driven universe.
Russell’s ideas were rooted in his belief that everything in the universe is interconnected through energy, and that the elements themselves are not static, but in a constant state of flux. This view was revolutionary at the time and has continued to inspire alternative theories in physics and chemistry.
The Traditional Periodic Table vs. Walter Russell’s Model
Before we explore Walter Russell’s Periodic Table, let’s take a look at the traditional periodic table. In 1869, Russian chemist Dmitri Mendeleev arranged the elements based on their atomic masses and observed periodic trends, such as the repeating nature of properties across different rows and columns. This organization was a breakthrough for understanding chemistry and was the foundation for modern chemistry as we know it.
However, despite its success, Mendeleev’s table still has limitations:
- It does not fully explain the forces behind atomic structure.
- It treats elements as fixed entities rather than dynamic ones.
- It doesn’t account for some of the more esoteric relationships between elements.
Walter Russell’s periodic table, in contrast, offers a new perspective by organizing elements based not only on their atomic mass but also on a deeper understanding of their underlying energetic properties. His approach emphasizes cycles, harmonics, and the interplay between matter and energy.
Key Features of the Walter Russell Periodic Table
The Walter Russell Periodic Table introduces several novel features that differentiate it from the traditional model. These features are inspired by Russell’s vision of the universe as a dynamic, energy-driven system.
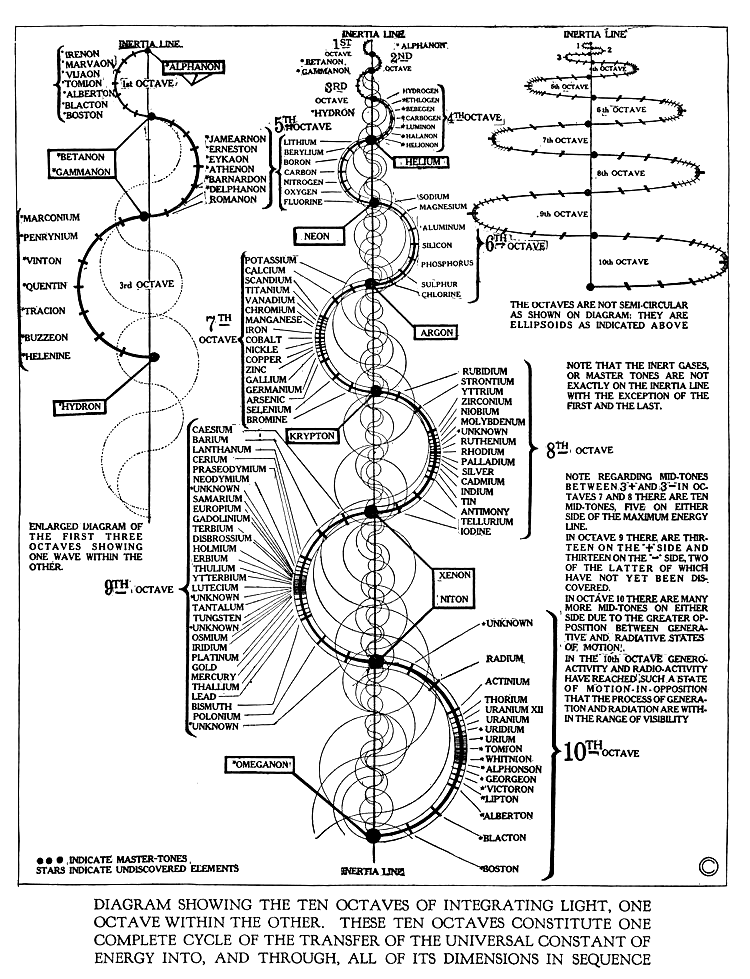
The Concept of “Octaves”
One of the most striking aspects of Russell’s table is his concept of “octaves.” In music, an octave refers to a doubling of frequency, which produces a harmonious relationship between two notes. Russell applied this principle to the periodic table, suggesting that the elements are arranged in octaves of energy. This means that each “octave” represents a specific energy level or vibrational frequency.
For example:
- The first octave of elements (starting with Hydrogen) represents the lowest vibrational energy.
- The second octave begins with Lithium, which has a higher vibrational energy.
This theory of octaves challenges the traditional idea of simply grouping elements based on atomic mass or number. Instead, Russell viewed elements as having specific energy signatures that corresponded to their position in the octave.
Spiral Structure
Russell’s periodic table is arranged in a spiral rather than a flat, grid-like structure. This spiral is not only a visual representation but also a deeper reflection of the interconnectedness of the universe. According to Russell, the elements spiral outward from a central point, each one progressing along a path of increasing complexity and energy.
The spiral concept embodies Russell’s belief in the cyclical nature of all things—where every cycle is both a repetition and an evolution of the previous one.
Two Spheres of Creation
Russell’s periodic table also divides the elements into two “spheres” or regions: one representing the elements of creation (which include light and positive energy) and the other representing elements of dissolution (which represent dark matter and negative energy). These spheres reflect Russell’s dualistic view of the universe, where forces of creation and destruction exist in harmony and balance.
The Role of “Inert Gases”
In Russell’s model, inert gases (like helium, neon, and argon) are not simply placed at the end of the periodic table but are considered central to the dynamics of the entire system. These gases, he suggested, play a critical role in balancing the energies of the other elements.
How the Walter Russell Periodic Table Relates to Modern Science
Though the Walter Russell Periodic Table is not universally accepted within the scientific community, it offers intriguing ideas that have inspired future generations of researchers. Some aspects of Russell’s theory have found resonance in more modern scientific fields, particularly in areas like quantum physics and energy dynamics.
- Quantum Mechanics: Russell’s emphasis on energy levels and vibrational frequencies aligns closely with principles in quantum mechanics. The idea that elements are not static but in constant flux mirrors the behavior of particles at the quantum level.
- Energy and Frequency: Russell’s concept of energy octaves can be compared to modern discussions of atomic energy levels and electron configurations. The notion of elements as vibrational frequencies echoes ideas in fields like molecular biology, where energy patterns are integral to the behavior of biological systems.
Although Walter Russell’s Periodic Table hasn’t been widely adopted by mainstream science, many researchers find his ideas about energy, creation, and dissolution to be a fascinating lens for understanding the universe.
The Influence of Walter Russell’s Ideas on Alternative Science
Russell’s work has inspired a wide range of alternative scientific thinkers. His theories extend beyond chemistry and physics into fields like free energy, sacred geometry, and cosmology. His contributions to the idea of an interconnected universe where energy flows freely and harmoniously have resonated with those exploring the boundaries of conventional science.
- Free Energy: Russell’s theories about energy cycles have inspired researchers in the field of free energy, where scientists aim to create devices that tap into the energy of the universe rather than relying on traditional power sources.
- Sacred Geometry: The spiral arrangement of elements in the Walter Russell Periodic Table shares similarities with sacred geometry, which explores the patterns found in nature and the cosmos. Many people who study sacred geometry have drawn parallels between Russell’s models and the natural world.
These intersections of Russell’s theories with other fields highlight the breadth and depth of his thinking, demonstrating that his work still holds value for those exploring alternative approaches to science.
Understanding the Walter Russell Periodic Table in Today’s Context
For those who are looking to understand how the Walter Russell Periodic Table fits into the broader context of modern science, here’s a simplified overview of how its ideas can complement and enrich current scientific thinking:
- Dynamic Approach to Chemistry: Instead of viewing elements as fixed entities, Russell’s table treats them as dynamic, energy-based systems. This shifts our understanding from a rigid scientific model to a more fluid, interconnected one.
- Holistic Understanding: Russell’s table encourages a more holistic approach to studying the periodic nature of elements. It suggests that chemistry is not isolated from the rest of science, but intertwined with physics, energy, and even consciousness.
The Walter Russell Periodic Table invites us to view science through a wider lens, one that sees the universe not just as a mechanical system, but as a living, evolving entity.
Conclusion: The Legacy of Walter Russell’s Periodic Table
The Walter Russell Periodic Table continues to captivate the imaginations of students, scientists, and thinkers across the world. While it may not yet be fully embraced by the scientific community, its innovative approach to understanding the elements provides us with a new framework to explore the relationship between matter, energy, and the universe.
As we continue to advance in our scientific pursuits, it’s worth remembering that groundbreaking ideas, such as those presented by Walter Russell, often challenge conventional wisdom. His contributions, particularly his reimagining of the periodic table, demonstrate the power of creative thought in the world of science.
By embracing new perspectives like those found in the Walter Russell Periodic Table, we move closer to understanding the intricate and beautiful relationships that govern the natural world. Who knows what future discoveries await as we continue to explore these fascinating ideas?
In summary, the Walter Russell Periodic Table offers a fascinating alternative to the traditional model, with its focus on energy, cycles, and interconnectedness. Its innovative approach has inspired numerous researchers and thinkers, making it an essential concept for anyone interested in alternative science.





